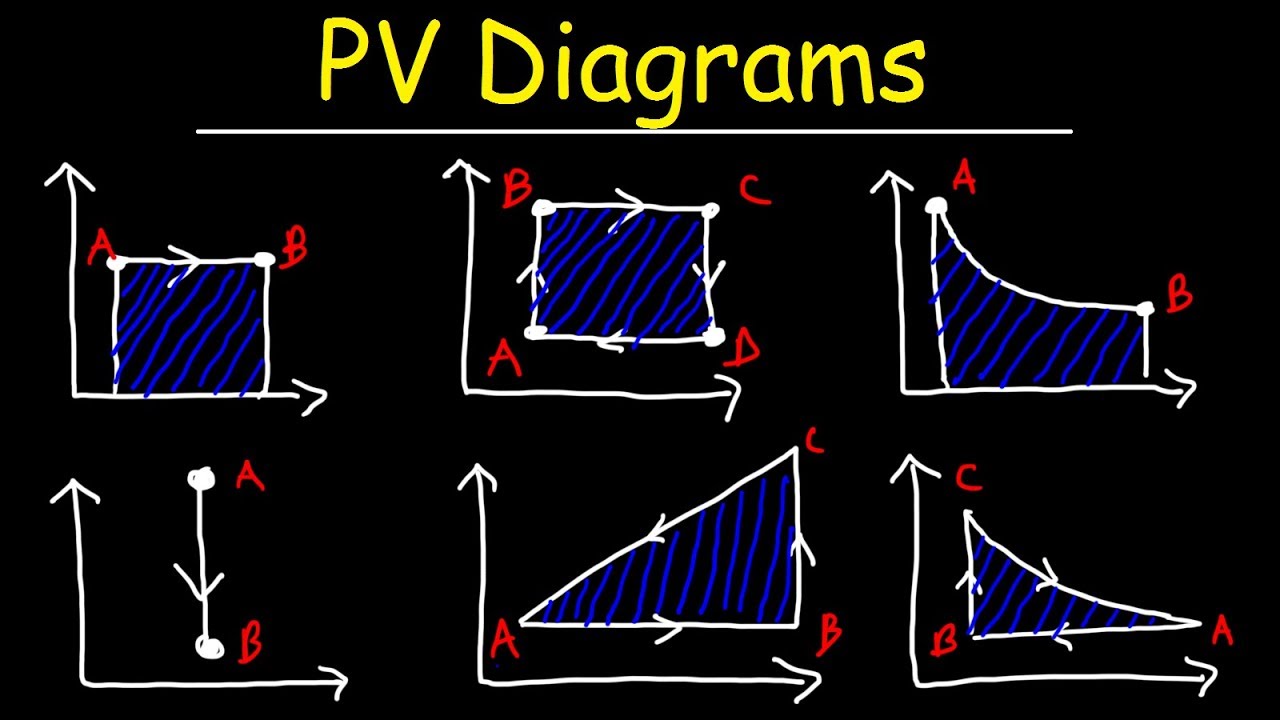
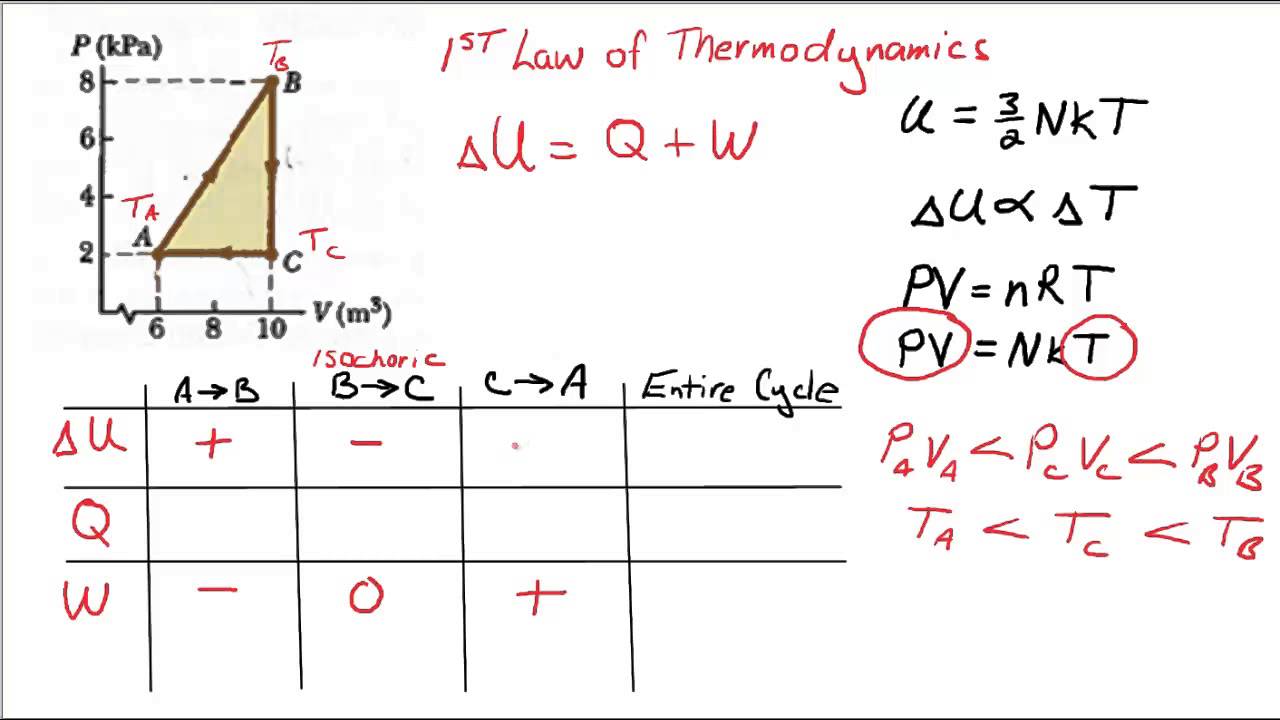
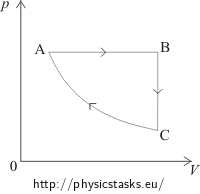
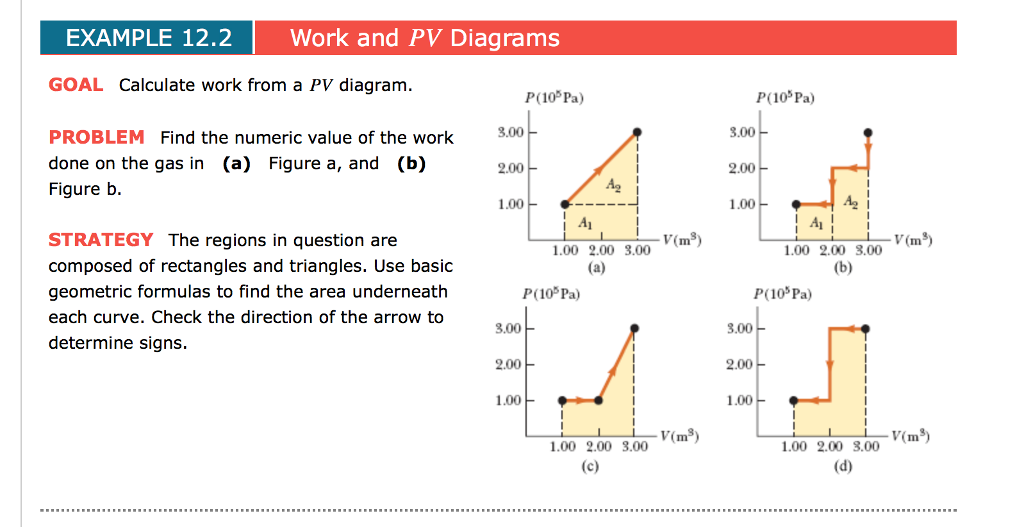
Pv Diagram Example Problems

Why heat is not a state function and internal energy is a state function.
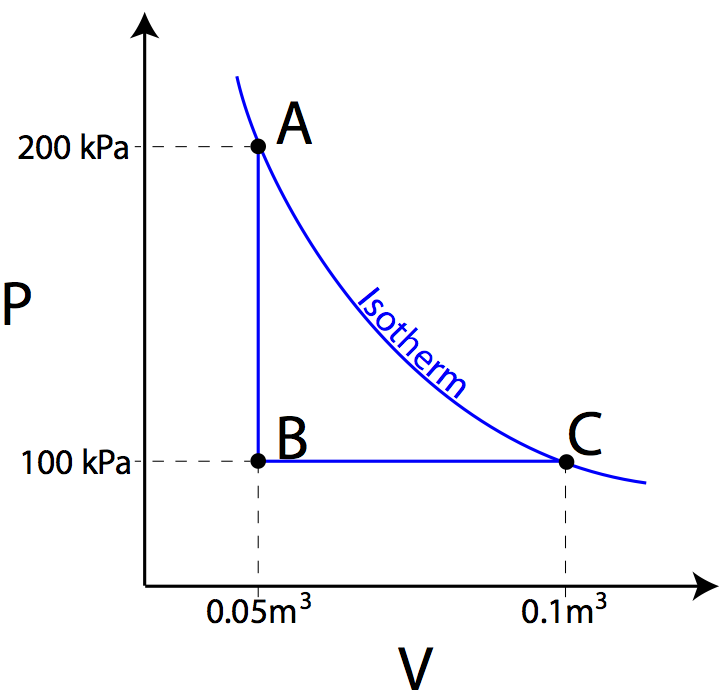
Pv diagram example problems. The pressure and volume of the gas at the extreme points in the cycle are given in the first two data rows of the table below. Example 126an adiabatic expansion the problems are at the bottom the rest is reference as given for the problem the pv diagram of an adiabatic expansion the graph of p cv g where c is a constant and g c p c v. A zero b positive c negative d cannot tell from the diagram 1b the processes from states 1 to 2 and 3 to 4 are. All processes are either isobaric or isochoric.
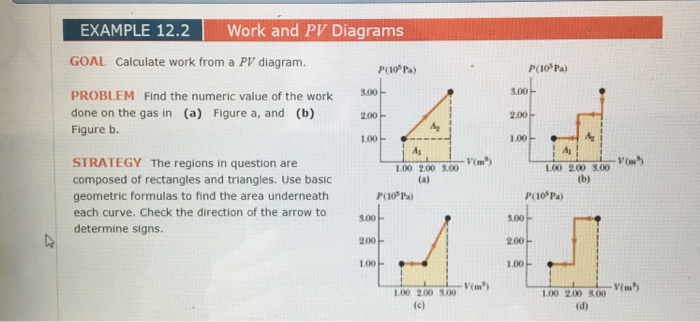
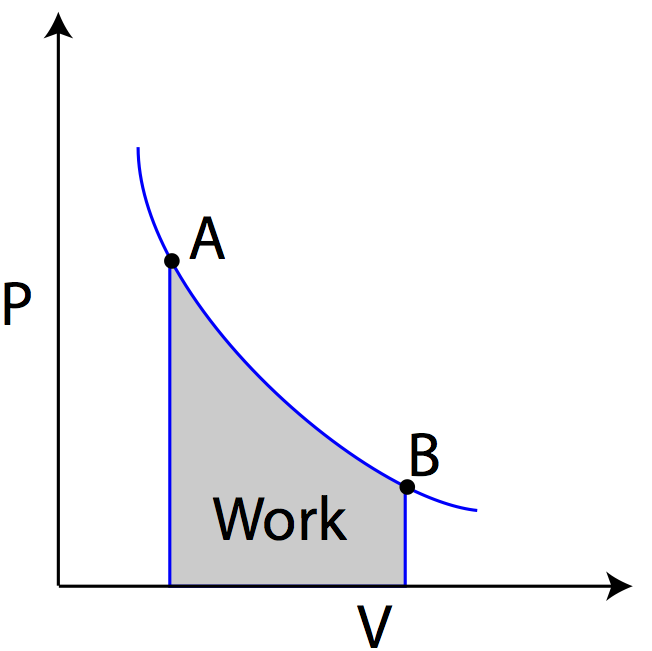
Pv g constant. Learn what pv diagrams are and how to use them to find the change in internal energy work done and heat. Sketch the cycle path on a pv diagram b calculate the net work done in kj data. Lecture 3 examples and problems reading.
Well maybe its only two variables. Practice problems chapters 1 4 1. Pv diagrams from the last section you were probably wondering what happens when we do something like add heat. Diagram and also pretty easy to understand from an example of a situation in which it is happening.
T 1 145 o c t 2 440 o c p 1 150 kpa. Many problems but it is especially helpful in adiabatic processes. Quasistatic and reversible processes. With everything tied together by the ideal gas law one variable can always be described as dependent on the other two.
Assume the co 2 behaves as an ideal gas throughout all three process steps. Work your way around the cycle step by step. Use the p v diagram below to answer the following questions 1a the net work for the cyclic process is. Ideal gas p v p t diagrams nkt p v increasing t volume pressure p vs v at various constant ts 0 pressure temperature 0 pressure zero as t absolute zero because the thermal kinetic energy of.
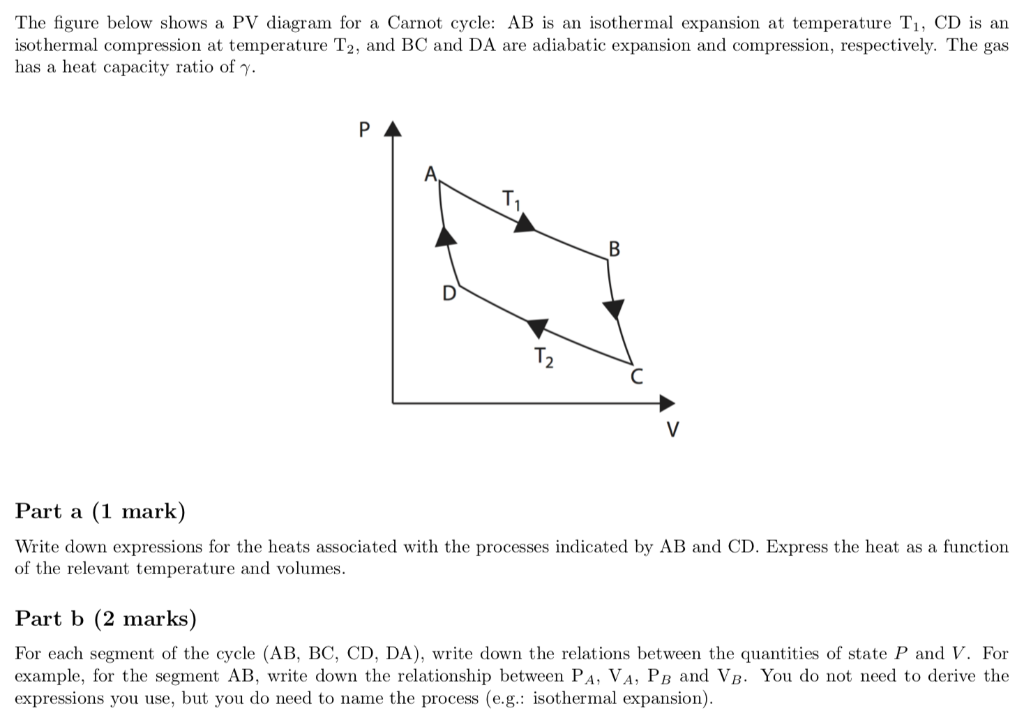
A system can be described by three thermodynamic variables pressure volume and temperature. What are pv diagrams. The work for the cycle is the sum of the work for each step. One mole of an ideal monatomic gas runs through a four step cycle.
More on internal energy.